What is in this article?
Atoms are the basic building blocks of all matters and the universe. Humans have long struggled to grasp both the very huge macro scale, such as the universe, and the micro scale, such as an atom. There are many mysteries that mankind still does not completely comprehend, hidden over immeasurable distances and small structures that are hard to find even with microscopes. But atoms are visible everywhere in the universe.
Today, we know that quarks and leptons, which make up protons, neutrons, and electrons, exist despite the presumption that atoms are the lowest indivisible building blocks of matter. There is also discussion about strings, which may represent even smaller building blocks. Together, these so-called subatomic particles—quarks and leptons—form electrons, protons, and neutrons. For instance, protons are made up of two up quarks and one down quark, whereas neutrons are made up of two down quarks and one up quark (energy.gov, 2022). Not only that, but interesting developments have happened, rendering quantum mechanics relevant and conventional physics obsolete on such a small scale.
First, let’s examine the definition of an atom and how the concept of an atom has changed throughout time.
What is an Atom?
Atoms, known as the smallest building blocks of matter, are the smallest units that exhibit the characteristics of a chemical element. Atoms are measured in Angstroms. One angstrom is the ten billionth part of a meter. The building blocks of the water molecule, which is necessary for all life, are hydrogen and oxygen atoms. Oxygen and hydrogen are also elements.
The elements listed in the periodic table are pure substances made up of only one type of atom. An element’s atom always has the same number of protons. Thanks to this characteristic feature of elements, the number of protons are also used as the atomic number. The atomic numbers of the elements determine where they are located in the periodic table.
What Makes Up Atomic Structure?
Three distinct subatomic particles—protons, neutrons, and electrons—are found in extremely tiny atoms. Quarks and leptons, two much smaller structural components, make up these subatomic particles. Beyond these particles, atoms consist mostly of empty space.
Positively charged protons and neutral, or uncharged, neutrons make up an atom’s nucleus. In general, the nucleus has a positive charge. Electrons with negative charges are orbiting around this positively charged nucleus.
An element’s nucleus always has the same amount of protons, while the number of neutrons varies. Atoms of an element consisting of different numbers of neutrons are said to be isotopes of each other. There may be several distinct isotopes for an element. The isotopes may have features that differ from the element’s natural state, such as radioactivity.
A neutral atom contains an equal number of protons and electrons in its nucleus. So, it is stable in terms of charge. Atoms can gain or lose electrons via collisions or chemical reactions. This electron transfer process is called ionization. Atoms that lose or gain electrons are known as ions. Because it has more positive charge, an atom that loses electrons is known as a positive ion, while an atom that receives electrons is called a negative ion.
Who Invented the Atom? The History of Atom

It was first proposed by Greek philosophers in the fifth century BC that matter is made up of extremely microscopic particles. Democritus believed that the matter has a finite and indivisible part rather than having an infinite structure. Democritus named this indivisible building block “atomos” from the Greek, which means indivisible, based on this notion.
Scientific research on the atom accelerated in the late 18th and early 19th century. John Dalton was the first to address the atom from a scientific standpoint with his atomic model. Dalton’s model, which was successful in drawing the scientific community’s attention back to this area, was frequently refuted, but it remains crucial since it opened the door for more research.
Through the efforts of scientists like JJ Thomson, Ernest Rutherford, Niels Bohr, Erwin Schrödinger, today’s modern atomic model was developed in the following years.
Atomic Models and Their Properties
Since the Ancient Greek philosophers first considered the fundamental units of matter, dozens of theories about atoms have been proposed. When scientists looked at atoms from a scientific standpoint, they proposed a number of theories between the 1800s and the present.
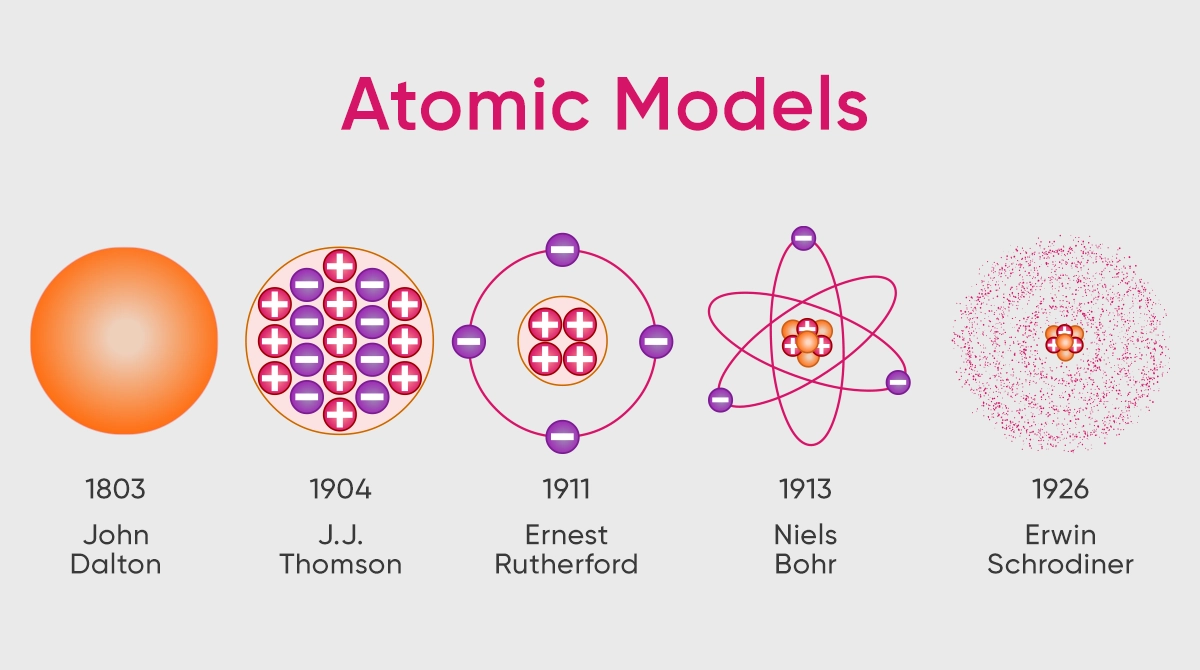
Dalton’s Atomic Model
Since considered a scientific approach, John Dalton’s atomic model, which was first introduced in 1803, is regarded as the first atomic theory. As per the theory of Dalton, the smallest building blocks of matter are extremely small and indivisible particles named atoms.
Dalton believed that these atoms have a solid structure, like a billiard ball. These moving atoms interact by colliding, resulting in chemical reactions.
This theory, which was deficient in understanding the internal structure of the atom, proved to be inadequate in many respects, including the belief that the atom could not be divided into smaller pieces as scientific advancements occurred in the following years.
JJ Thomson’s Atomic Model
J.J. Thomson’s atomic model which was introduced in 1904, and which claimed that atoms are a positively charged medium with negatively charged electrons, came one hundred years after John Dalton’s model. This model is also called “plum-pudding” model. The atom may be compared to a positively charged pudding, with negatively charged electrons placed inside the atom like grapes.
For the discovery of electrons, one of the subatomic particles, Thomson’s work is crucial. It was suggested by Thomson that atoms should have a neutral structure with equal quantities of positive charge. Thomson’s research with cathode rays led to the discovery of electrons, negatively charged particles.
The model’s shortcomings include its inability to account for protons and its inability to explain concepts like radioactivity, isotopes, and atomic stability.
Rutherford’s Atomic Model
The scientific community was working feverishly on the atom when Ernest Rutherford proposed his own atomic model, also known as the planetary model, in 1911. In his experiments, Rutherford aimed positively charged particles at a thin sheet of gold foil. The majority of the particles passed through the gold foil, and a very small number of these particles deflected at wide angles.
As a result of his work, he concluded that there must be a small, highly dense and positively charged nucleus in an atom. Moreover, he assumed that negatively charged electrons were orbiting around the nucleus in a manner similar to how planets orbit around the sun.
However, Rutherford’s model did not take into account the wave-like structures of electrons. Also, the model was inadequate in explaining the movement of negatively charged electrons around positively charged nucleus.
Bohr’s Atomic Model
Only two years after Rutherford’s work, in 1913, Niels Bohr proposed his own atomic model, developed based on Rutherford’s findings. In his atomic model, Bohr expanded on Rutherford’s research and proposed that electrons orbit the nucleus in several distinct directions. He argued that the energy level of an electron determines where it is located in each of these several orbits.
While Bohr’s atomic model and the modern atomic model are most comparable, Bohr’s model is not entirely correct. When it came to atoms with basic structures, like hydrogen, Bohr’s model appeared to be accurate; nevertheless, as atomic structures got more complicated, it began to function incorrectly. It is also inadequate in terms of behaviors and movements of electrons. However, Niels Bohr’s work laid the foundations of the modern atomic model.
Modern Atomic Model
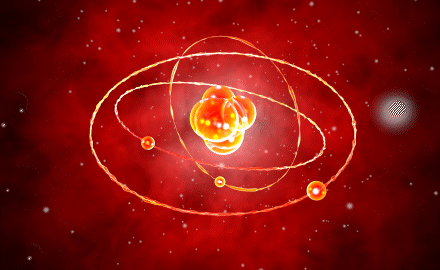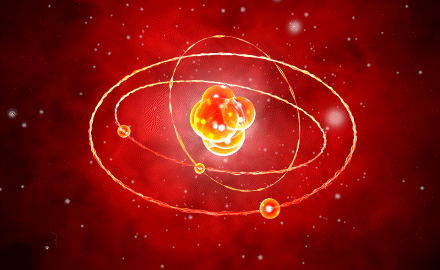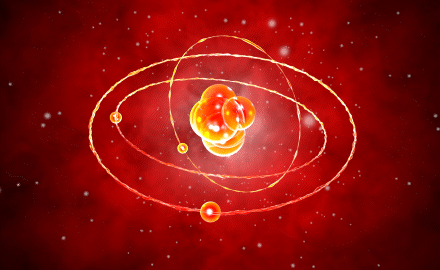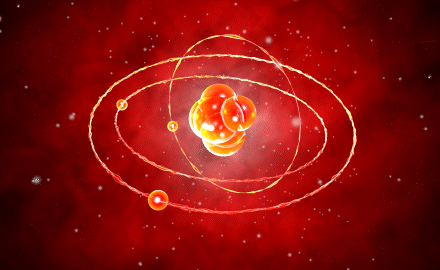
The model by Erwin Schrödinger introduced in 1926, also known as the quantum mechanical model, contained the solutions to the missing components of every model developed up to that point. This model explained the positioning of electrons in orbits, so addressing the inadequacies of Bohr’s model.
The model, in which electrons are demonstrated to have a wave character, proposes that electrons can settle in orbits that are integer multiples of the electron wavelength. This model mentioned an electron cloud surrounding the nucleus since it is impossible to pinpoint the exact location of the electrons.
Subatomic particles known as quarks and leptons have been extensively studied in the years that followed due to scientific discoveries. It is believed that these subatomic particles are made up of smaller components known as strings as a consequence of research in the field of quantum mechanics.
Current Studies on Atoms
Studies on atoms have improved our understanding of the universe and sparked the development of several useful uses in daily life. It has been discovered that atoms’ nuclei, which have long been thought of by humans as the indivisible fundamental building blocks of matter, are capable of splitting up or merge with other nuclei.
The process of two elements combining to form a heavier element as a result of nuclear reactions is known as nuclear fusion. The main energy source for the sun and stars is the nuclear fusion reactions that take place spontaneously in these celestial entities. Scientists mimic these processes in an effort to produce energy.
A nuclear fission process is defined as the splitting of an atom’s nucleus. Large nuclei of atoms break apart to generate smaller nuclei, resulting in the formation of new atoms. An enormous amount of energy is produced during this process. Nuclear or atomic power plants and atom bombs developed with the leadership of Robert Oppenheimer also use nuclear fission reactions.
Atoms are the building blocks of molecular biology and nanotechnology in addition to nuclear physics. Nanotechnology manipulates atoms and molecules to modify their characteristics in order to study things at the nanoscale. The field of biology known as molecular biology and genetics seeks to comprehend the molecular structure of cells.

Particle accelerators are being used in many studies to discover more about atoms and the structure of the universe. Furthermore, our understanding of atoms has aided in the creation of atomic force microscopes and nanotechnology, which allow for nanoscale imaging and material processing. Research in the field of molecular biology has yielded knowledge on the nature and mechanisms of living creatures. All of this work around the atom serves as the foundation for civilizational progress and the expansion of human life-span. We observe the results of this scientific research in many different aspects of daily life, such as food production, medical imaging and therapies, and energy generation.
The behavior of atoms and subatomic particles, which is difficult to observe even with microscopes, doesn’t it pique your curiosity and excite you? Don’t forget to share your comments regarding this subject.

 Online Services
Online Services Application Inquiry
Application Inquiry Pay Assurance Fee
Pay Assurance Fee Query Installation Number
Query Installation Number Compensation Fee Inquiry
Compensation Fee Inquiry Automatic Payment Order Inquiry
Automatic Payment Order Inquiry Partnership
Partnership







Leave a Comment