What is Photoelectric Effect? History and Areas of Use
What is in this article?
Since ancient times, the nature of light has been one of the most intriguing topics. The photoelectric effect, first discovered by Heinrich Hertz in 1887, is a scientific approach that explains the structure of light. It is often used in fields such as electrochemistry and electronic physics.
Dive into our blog on the photoelectric effect, a pivotal topic in modern physics, to uncover its fascinating discovery and its applications in daily life and scientific research.
What Is a Photoelectron?
Photoelectrons are electrons that are released from the structure of substances when exposed to light. In other words, they are electrons that break off as a result of the impact of a photon (a particle symbolizing light or electromagnetic radiation species) on the radiating surface.
In various scientific studies examining the nature of light, some posit that light can be described by its wave-like properties, while others advocate for its particle-like characteristics. However, with the development of quantum physics, it has become accepted that light has both a particle and a wave structure.
The photoelectron is an important step in explaining light using the particle model. With the discovery of this effect, the nature of light transitioned from the realm of classical physics to modern physics.
Observations and scientific studies of the photoelectron, first by the German physicist Heinrich Heart and later by Albert Einstein, made it possible to explain the nature of light with modern physics. The photoelectric effect is one of the pivotal discoveries in 19th-century physics.
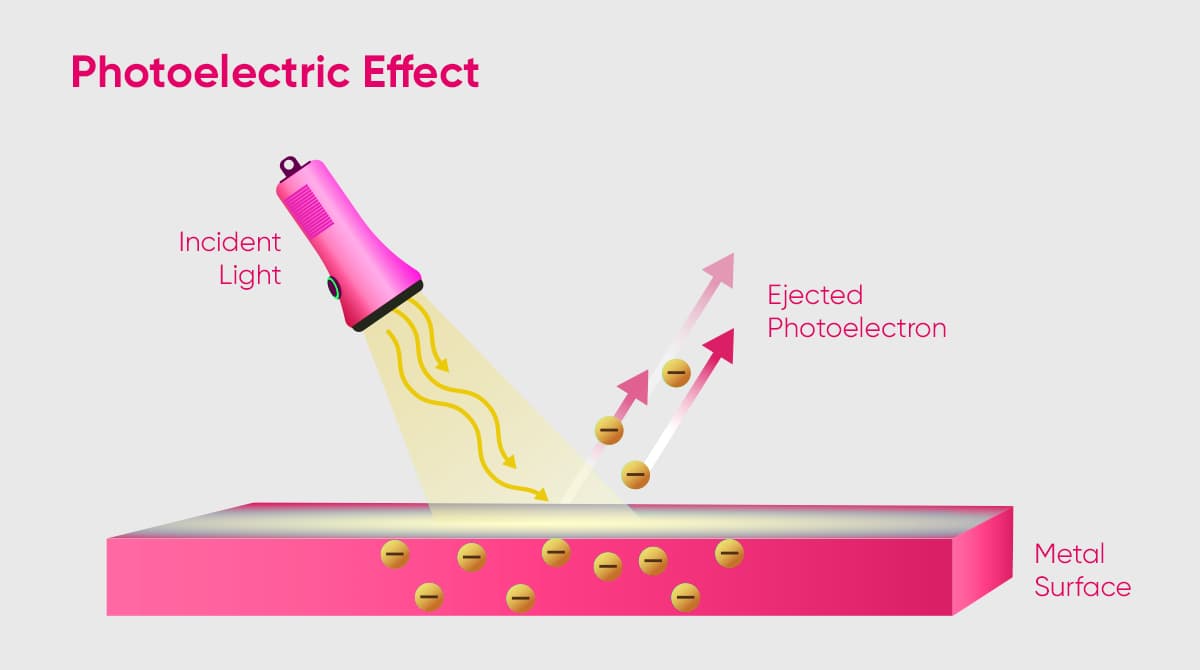
What is Photoelectric Effect?
The photoelectric effect is the phenomenon of the detachment of electrons as a result of light striking the surface of a material. Photoemission usually occurs in metals and other conductive materials.
In general, metals have a frequency threshold that varies from substance to substance. The photoelectric effect occurs when light, with energy exceeding the threshold frequency of a metal, strikes the surface of a material.
When light reflects off the surface of a material, it impinges on the electrons within the material's structure and transfers its entire energy to them. The energy transferred to the electron causes the particle to detach from the substance. This process is termed detachment of the electron from the atomic structure of the material due to the influence of light. In physics, the photoelectric effect is also called photoemission.
-
Mathematical Description of Photoelectricity
In the photoemission phenomenon described by Einstein, light with frequency f is conceptualized as a packet of photons.
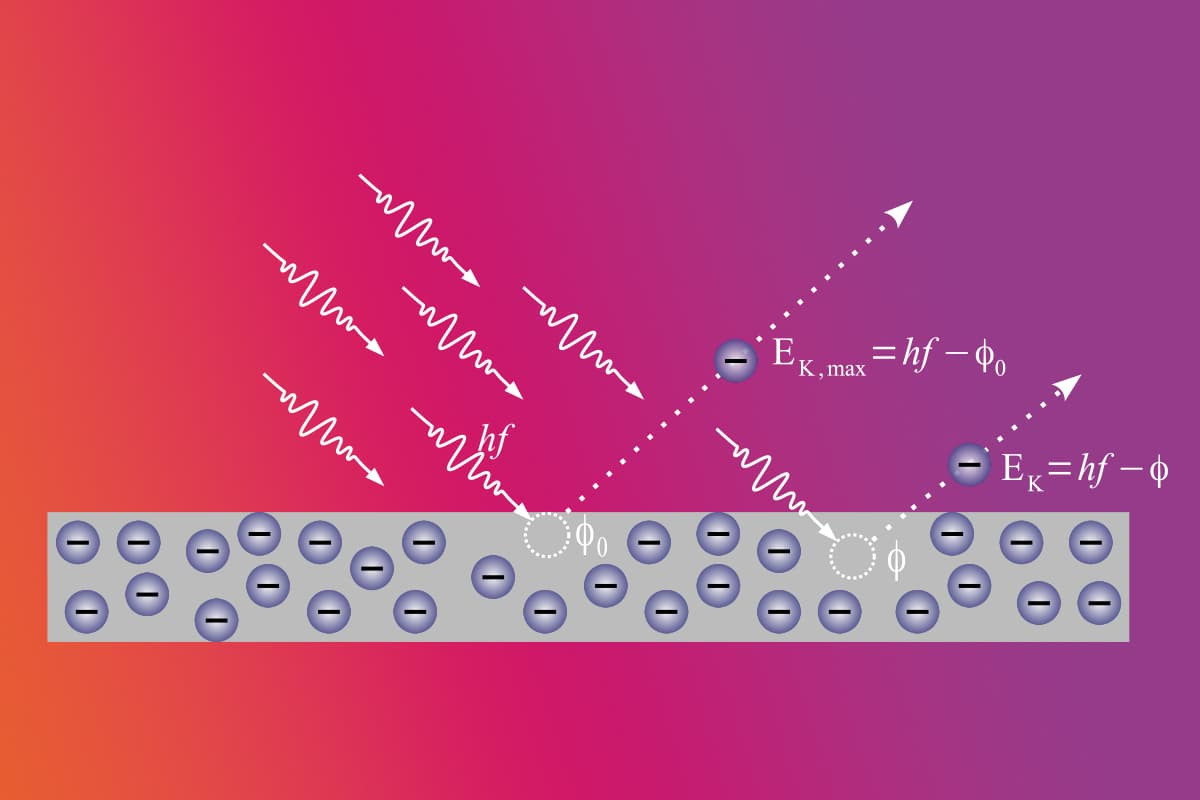
Based on the principle that each photon has an energy E, it was assumed that the electrons detached from the metal surface had a kinetic energy of Kmax. Based on Einstein's formula for the E energy of the photon E= hf, the following equation was used to determine the kinetic energy of the electrons.
Kmax= hf - Ø
- Kmax is the maximum kinetic energy of the electrons emitted from the surface.
- The hf in the formula is defined in physics as Plank's constant. Plank's constant refers to the frequency of the photon hitting the metal surface.
- The symbol Ø can be defined as the minimum threshold frequency required for the electrons to detach from the metal surface.
These two values are needed to determine the maximum energy of the electron detached from the metal. The mathematical definition of photoelectricity provides a formulation of the phenomenon in physics.
Bonus: Photoelectric Effect Experiment
History of the Photoelectric Effect
Research into the photoelectric effect commenced in the 19th century, with early efforts seeking to define the nature of light through the lens of classical physics.
 The foundation for the pioneering studies in this field was laid by the German physicist Heinrich Hertz.
The foundation for the pioneering studies in this field was laid by the German physicist Heinrich Hertz.
The famous physicist, in his experiment in which he irradiated a metal surface with light, observed that the light causes the electrons in the structure of matter to break.
However, this observation was not theorized within the framework of the laws of classical physics. A modern physics approach was needed to make sense of this phenomenon.
Following Heinrich Hertz's discovery in 1887, Philipp Lenard investigated the effect of light on gaseous matters in 1900. In his work, the famous scientist observed that gases ionized under ultraviolet light, resulting in an increase of positively charged ions in the structure of matter.
The data obtained from these experiments could not be explained by electromagnetism within the framework of classical physics. According to the electromagnetic approach of classical physics, the nature of light was assumed to be a wave.
The reaction of metals and gaseous substances under the action of light in 1887 and 1900 pointed to the particle nature of light.
Building on the research of Heinrich Hertz and Max Planck regarding light, Albert Einstein formulated the photoelectric law. This effect belongs to the important stages of modern physics.
The history of the photoemission effect can be described as follows:
- 1887- Heinrich Hertz's discovery that the photon causes electron fracture when it strikes metallic materials
- 1905- Albert Einstein's formulation of the photoelectric effect
- 1914- Robert Milikan confirms Einstein's law of the photoelectric effect
- 1921- Albert Einstein's "Law of the Photoelectric Effect" is honored with the Nobel Prize
- 1923- Robert Milikan's scientific work on the photoemission effect and the basic electric charge is honored with the Nobel Prize
Studies on photoemission formed the basis for the transition to modern physics. In the 19th and 20th centuries, the theories of classical physics were replaced by quantum theory as science evolved.
Related Article

Who Invented Electricity? History of Electricity
Albert Einstein and Photoelectricity: The Discovery That Won the Nobel Prize
In 1905, Albert Einstein, one of the most important scientists of the 20th century, described the photoelectric phenomenon based on the quantum theory proposed by Max Planck.
 His new observations on photoemission led to important developments in the world of physics and were among the seminal studies of modern physics. Thus, in 1921, Einstein made the discovery that earned him the Nobel Prize: the Law of the Photoelectric Effect.
His new observations on photoemission led to important developments in the world of physics and were among the seminal studies of modern physics. Thus, in 1921, Einstein made the discovery that earned him the Nobel Prize: the Law of the Photoelectric Effect.
Contrary to classical physics, Einstein described the detachment of electrons from the structure of matter due to light striking a metal surface—specifically, the beam of photons—using the particle nature of light. Einstein argued that light is not a wave, but consists of a beam of photons.
The scientist deduced from his experiments that photons possess a frequency, and when this frequency meets or surpasses the threshold value, electrons are detached upon striking the surface of metallic materials.
When the frequency of the photons is below the threshold and hits the metal surface, no electrons are emitted from the material. The detachment of electrons from a metal material is contingent upon the photon's frequency reaching a specific threshold.
The intensity of light or the duration of exposure to the substance has no influence on the detachment of electrons. After Einstein's discovery, it was explained that light is not a wave propagating in space.
 It was found that light consists of different photon beams with hf energy. The hf energy of photons is a discovery in the field of physics previously described by Max Planck. Einstein based the photoelectric effect on Max Planck's discovery.
It was found that light consists of different photon beams with hf energy. The hf energy of photons is a discovery in the field of physics previously described by Max Planck. Einstein based the photoelectric effect on Max Planck's discovery.
Einstein's pivotal theory on photoemission posits that the nature of light cannot be elucidated solely by the electromagnetic laws of classical physics. He thus laid the foundation for new developments in modern physics. The electromagnetic theory of classical physics explained the structure of light in terms of waves.
In classical physics, the dominant view is that the electromagnetic effect provides for the transfer of energy from light to the electron. For this reason, the wavelength and intensity of light also cause differences in the amount of electrons released from the metal.
However, experiments and scientific investigations led by Einstein demonstrated that this classical viewpoint was incorrect, and the detachment of electrons from metallic materials could indeed be explained by the photoelectric effect.
Einstein's law of the photoelectric effect is based on three basic principles:
- The kinetic energy of the photoelectron is directly proportional to the frequency of the photons.
- When the frequency of the photons is above the threshold value, detachment of the electrons from the metal occurs regardless of the intensity of the light.
- As long as the frequency of photons in the light structure does not reach the threshold, electron detachment does not occur, no matter how long the metal object is exposed to high intensity light.
Related Article

What is Quantum Mechanics?
Areas of Use of the Photoelectric Effect
The photoelectric effect is used in image sensors, televisions, and cameras. The photoelectric effect is also fundamental in devices like photodiodes (light-sensitive elements that allow current to flow in one direction) and phototransistors (semiconductor devices whose conductivity changes in response to incident light).
In devices that utilize the photoelectric effect, the principle at work is that when a photon strikes the material, it excites electrons in the valence band (the frequency range required for an electronic device to operate), generating an electric current.
Image Sensors
The photoelectric effect was harnessed in the video camera tubes of early television models.
In contemporary television designs, photoemissive and photoconductive substances are employed. It can be argued that the photoemission effect had an important influence on the invention of television. The operating principle of image sensors is based on the photoelectron effect. This effect is also used in various imaging devices such as cameras.
Phototransistors and Photodiodes
The photoemission effect is instrumental in the functioning of light-sensitive diodes, solar cells, and batteries. In such devices, even under low light intensity, photons induce electron movement within the valence band. As a result, these devices generate electric current.
In everyday life, light-sensitive diodes are utilized in the manufacturing of devices like smoke detectors. The device, called an electronic eye, exhibits different electrical properties depending on the incidence of light.
When smoke enters the detector, the photocell's light particles propagate in the chamber and trigger the alarm. The photoemission effect is harnessed in the manufacturing of various security systems. Photocells incorporated into these devices enable their operation based on the photoelectric effect.
Phototube
Phototubes were one of the first things to tap into the photoemission effect. Phototubes act as a switch in an electrical circuit. When a photon with the right threshold frequency hits the metal, phototubes generate an electric current.
In the dark, when no photons hit the phototube, the circuit is closed and no current flows through the device. Phototubes can be used to read sound recordings in motion pictures and as sensors for security alarms.
Digital Scanner
Digital scanners capture text or images and save them to the memory of electronic devices like computers and phones. The scanned data can then be shared over the Internet or printed.
Devices like photocopiers and printers employ digital scanners to read data, such as documents and images, through the exposure to light. This operation of digital scanners is contingent on the photoemission effect.

What is Photoelectron Spectroscopy?
Photoelectron spectroscopy (PES) is a prime example of the photoemission effect's application in chemistry. This technique can be implemented in two distinct methods. Photoelectron spectroscopy techniques using ultraviolet or X-rays can be used to study many different properties of substances such as binding energies or percent distribution of atoms.
Photoelectron spectroscopy is a technique used to investigate the electron structure on or near the surface of solid materials. It is an experimental method for measuring the relative energies of electrons in the structure of atoms or molecules.
In these experiments, samples are exposed to high-energy X-rays or ultraviolet radiation, and the kinetic energy of the electrons detached from the atomic structure is measured. PES is based on the application of the photoelectric effect to molecules or atoms instead of metal objects. Experimental measurements taken using this technique can reveal the chemical structure of various elements, including oxygen and lithium.
PES is a technique commonly used in experimental methods in chemistry. The photoelectron emission spectrum is used to obtain information about the electronic levels of the atoms of the material. Through this method, attributes like atomic structures and binding energies of chemical elements embedded within a material can be pinpointed with great accuracy.
X-Ray Photoelectron Spectroscopy
Electron spectroscopy uses X-rays with photon energies between 100 eV and 10 keV, corresponding to a wavelength of 10- 0.1 nm, for chemical analysis. The analysis is performed at the deep nuclear levels of the atoms embedded in the substance.
Infrared (Ultraviolet) Photoelectron Spectroscopy
With electron spectroscopy, ultraviolet radiation with photon energies between 10 and 50 eV corresponding to a wavelength of 10- 25 nm is used for analysis. It is used for valence and conditioning band regions.
What do you think about the photoelectric effect? Share your opinions and comments on the contributions of photoemission in the world of science.

 Online Services
Online Services Application Inquiry
Application Inquiry Pay Assurance Fee
Pay Assurance Fee Query Installation Number
Query Installation Number Compensation Fee Inquiry
Compensation Fee Inquiry Automatic Payment Order Inquiry
Automatic Payment Order Inquiry Partnership
Partnership






Leave a Comment