
What is in this article?
Quantum mechanics opens up a window into the strange and incomprehensible world of the smallest particles. In our everyday lives, we rely on predictable laws to make sense of the world. However, when we delve into the realm of atoms and electrons, these familiar rules break down, giving way to a landscape of uncertainties and split identities.
While everything is in its place in the classical mechanical world, nothing is as it seems in the quantum world. Understanding matter and energy from this brand new perspective has had revolutionary effects on technology, science and philosophy.
Let's take a step into the details of the world of quantum mechanics, which inspires new generation technologies with its revolutionary impact.
What is Quantum Mechanics?
Quantum mechanics, known as one of the most complex and enigmatic branches of physics, studies how matter and light behave at the atomic and subatomic levels.
Also referred to as wave mechanics, this branch of science seeks to elucidate the characteristics of particles like molecules, atoms, and subatomic entities including electrons, protons, neutrons, quarks, and gluons. Additionally, it explores how these particles interact with various forms of electromagnetic radiation, such as light, X-rays, and gamma rays.
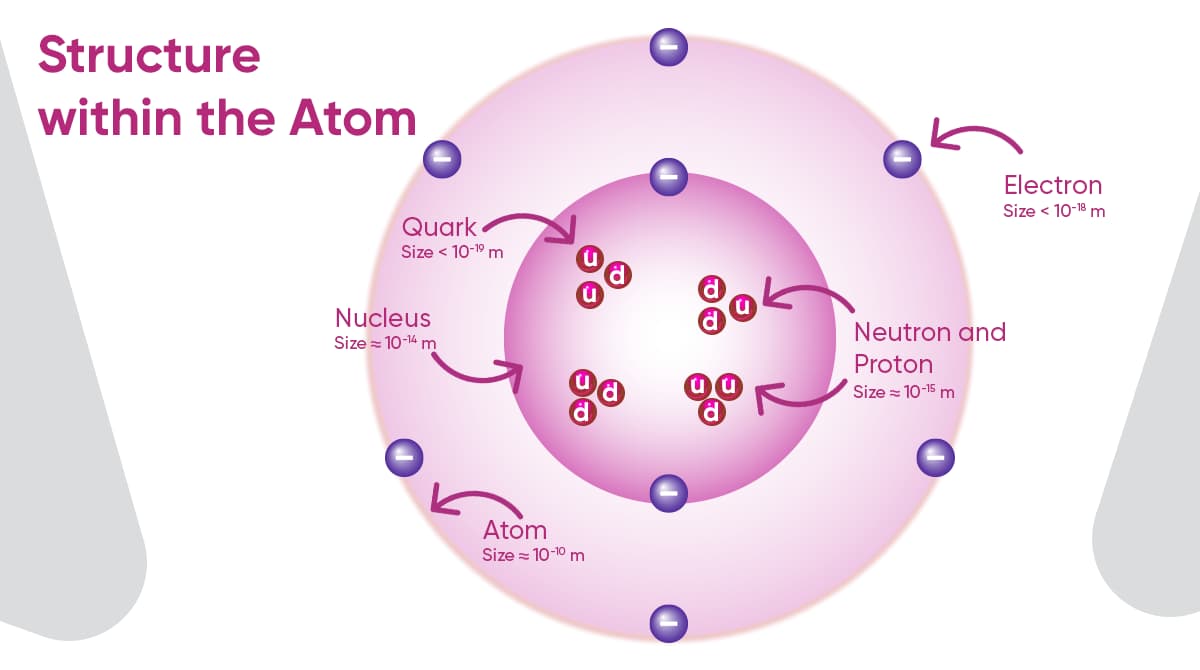
The name of quantum mechanics is derived from the Latin word quantus, which means “how much” or “how big.” In other words, it allows us to understand the nature of matter and energy, while showing us that some things in the universe are discrete (i.e., in the form of particular “particles” or “packets”).
For example, electromagnetic radiation (e.g., light) can be thought of as a continuous wave. However, quantum mechanics suggests that this radiation exists in individual packets of energy called photons. This means that light has both wave and particle properties.
-
History of Quantum Mechanics
In the late 19th century, quantum mechanics emerged as an alternative to classical mechanics to solve problems that could not be explained by statistical mechanics and thermodynamics.

The origins of this scientific discipline can be traced back to Max Planck’s groundbreaking 1900 paper on blackbody radiation.
Planck's work influenced scientists such as Albert Einstein, Erwin Schrödinger, and Werner Heisenberg, who later played key roles in the development of quantum theory.
In 1925, Schrödinger and Heisenberg established the mathematical underpinnings of quantum mechanics. They employed concepts from linear algebra and partial differential to analyze the structures of atoms and molecules.
While classical mechanics assumes that matter and energy are continuous, quantum mechanics assumes a discrete, i.e. quantized structure. From this point of view, particles are defined by certain states and probabilities in quantum mechanics. Moreover, this approach has succeeded in explaining phenomena that classical mechanics cannot.
Quantum mechanics has surpassed the constraints of classical mechanics, which struggled to accurately explain phenomena like blackbody radiation and the photoelectric effect in the early 20th century. Today, it serves as an essential framework in a wide range of disciplines, including physics, chemistry, engineering, and computer science.
This versatile branch of science is used in many areas of modern technology and industry. For this reason, it can be argued that the history of quantum mechanics is also the history of modern science and technology.
Who Contributed to the Theory of Quantum Mechanics?
Many scientists have contributed to quantum mechanics throughout history. These individuals are:
- In 1900, Max Planck proposed the revolutionary concept that energy exists in quantized amounts as a way to theoretically account for the phenomenon of blackbody radiation. This idea is considered the birth of quantum theory.
- In 1905, Einstein, significantly advanced quantum theory through his work on the photoelectric effect. He posited that light energy should be understood as quantized in the form of photons. The photoelectric effect refers to the emission of electrons from a surface when light (usually ultraviolet light) falls on it.
- In 1913, Niels Bohr, in explaining the structure of the hydrogen atom, showed that electrons can exist at different energy levels, just like nuclear energy.
- In 192, Werner Heisenberg developed the Heisenberg uncertainty principle. According to this principle, certain measurements cannot be made simultaneously with certainty.
- In 1926, Erwin Schrödinger developed a wave-mechanical interpretation of quantum mechanics. The Schrödinger equation describes how the wave function of a particle changes over time.
- In 1928, Paul Dirac developed the Dirac equation, which reconciles quantum mechanics with special relativity.
- Three physicians Richard Feynman, Julian Schwinger, and Tomonaga Shinichiro made significant contributions to the development of quantum electrodynamics (QED) in the 1940s.
- In the 1960s, Murray Gell-Mann and George Zweig developed the quark model. The model suggests that hadrons, like protons and neutrons, are made up of more elementary particles called quarks.
Wave and Particle Relationship
The wave-particle duality in quantum mechanics refers to the interesting phenomenon that light and matter can behave as both waves and particles, depending on the experimental conditions. Originating in the 1600s from attempts to comprehend the nature of light, the concept eventually evolved to encompass matter as well, revealing its dualistic properties. The work of scientists such as Einstein and De Broglie played an important role in understanding this duality.
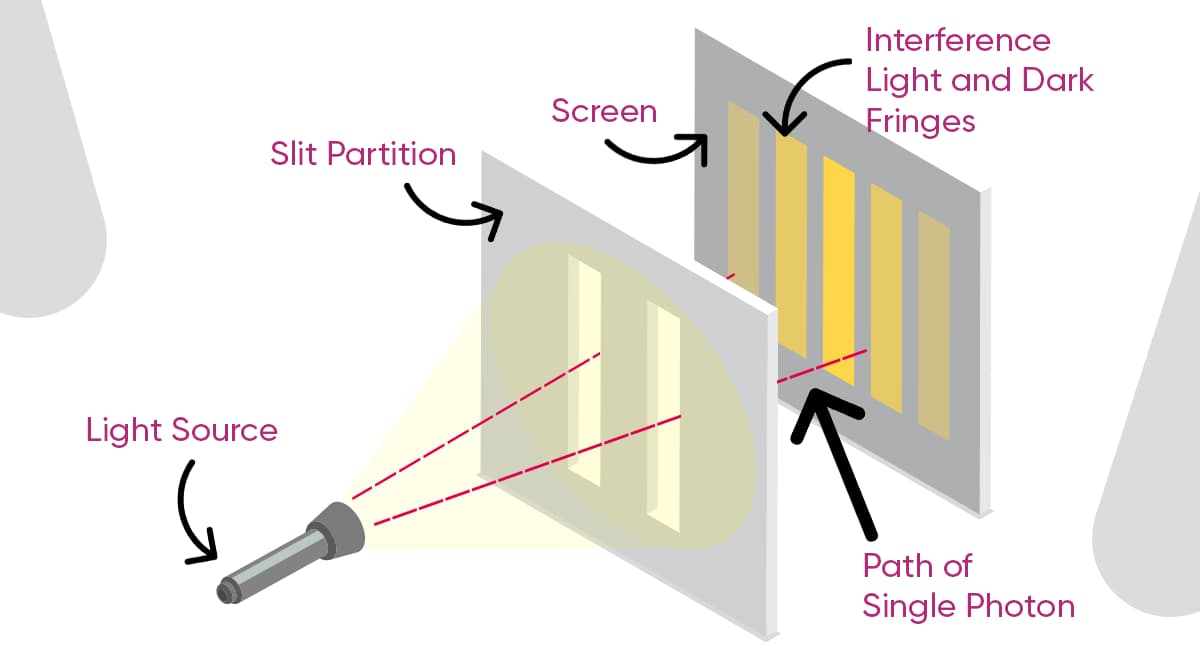
One of the most impressive visualizations of the wave-particle relationship is the double slit experiment. Some steps must be followed to conduct the experiment.
For example, take a light source (or particle source) and place the light (or particles) from this source in front of a barrier with two slits. When light —or particles— passes through these slits, they hit a screen located behind them.
In classical physics, if only one slit is open, you'd expect to see a single band or dot appear on the screen. Similarly, if both slits are open, you'd anticipate seeing two distinct bands or dots. However, the observed outcomes defy these classical expectations. Instead, one sees a series of dark and light bands created by the wave interaction of light. And this shows that light behaves like a wave. What's truly astonishing about this experiment is that a similar interference pattern emerges even when it is conducted with particles, like individual electrons. This shows that a single particle behaves as if it were passing through both slits simultaneously.
Wave-particle duality is one of the most amazing aspects of the quantum world. The dual nature of light—acting as both a wave and a particle in the form of photons—as well as the similar wave-particle duality observed in matter, remains one of the enigmatic facets of quantum mechanics that is not yet fully comprehended. While mathematical frameworks like the Schrödinger equation have been employed to shed light on this dual property, the phenomenon continues to be an active area of exploration and research.
Reflecting the complex nature of light and matter, wave-particle duality is a phenomenon that plays a fundamental role in understanding the physical world, but has yet to be fully comprehended. The concept highlights not only the bizarre nature of quantum mechanics, but also the complex relationship between observation and reality.
What is the Uncertainty Principle?
First formulated by Werner Heisenberg, the Uncertainty Principle is one of the fundamental aspects of quantum mechanics. The principle states that both the position and momentum (the magnitude and direction of an object's motion) of a given particle cannot be precisely measured at the same time. In essence, the more accurately one knows a particle's position, the less certain one becomes about its momentum. Conversely, the more precisely the momentum is determined, the greater the uncertainty in its position.
The Uncertainty Principle emphasizes the probabilistic nature of the quantum world. In classical physics, the position and velocity of an object can be precisely determined at the same time. But in quantum mechanics, knowing one more precisely makes the other more uncertain. On the other hand, the uncertainty principle reveals the complexity of the measurement concept in quantum mechanics and the inherent uncertainty of particles.
This uncertainty exists not only during measurement, but also as an intrinsic property of quantum systems. This principle reveals that quantum particles don't follow a predefined trajectory; rather, they are described by probability waves.
Are Quantum Mechanics and Conventional Physics at odds?
Quantum mechanics and traditional (classical) physics involve quite different structures and principles. Although at first glance this may seem contradictory, it actually reveals that the two fields are applied at different scales and under different conditions. These can be summarized as follows:
- Quantum mechanics describes behavior at very small scales, such as subatomic particles, while traditional physics is used for systems at the macroscopic scale. These macroscopic systems are much larger than the atomic or molecular level and are directly observable with the naked eye. In the quantum realm, extraordinary phenomena like uncertainty, superposition, and wave-particle duality come into play. These effects are not observed in the large-scale world we experience in our daily lives.
- When properly examined, quantum mechanics can also elucidate how the classical laws that govern the macroscopic world come into being. In large-scale systems, the unique effects seen in quantum mechanics are averaged out, resulting in the classical laws that we observe. In essence, while quantum mechanics is actually compatible with classical physics, it provides a broader and more general framework.
- Quantum mechanics challenges our philosophical notions about the nature of existence and observation through concepts like uncertainty and measurement. This stands in stark contrast to the precise and deterministic methodology that classical physics adopts. Because of these foundational differences, the two theories can seem to be at odds with each other at first glance.
- At the core of quantum mechanics lie mathematical tools like wave functions and the Schrödinger equation. These structures differ fundamentally from the Newtonian laws of motion that underpin classical physics.
- In the realm of classical physics, matter is described by definite positions and velocities, and under specific conditions, these variables can be precisely predicted. For instance, the motion of a planet in its orbit around the sun can be calculated with high accuracy. In other words, the motion of the object proceeds in a precisely determinable, predictable way. In quantum mechanics, the exact position and velocity of a particle cannot be known simultaneously (Heisenberg uncertainty principle). In contrast, quantum mechanics employs a wave function to describe the likelihood of finding a particle at a particular position or with a specific velocity. This means that an electron does not move in a particular orbit around an atom, but may be in many orbits at the same time. When a measurement is taken in the quantum context, one of these probabilistic outcomes comes true. However, before the measurement, the electron is in superposition on all possible orbits.
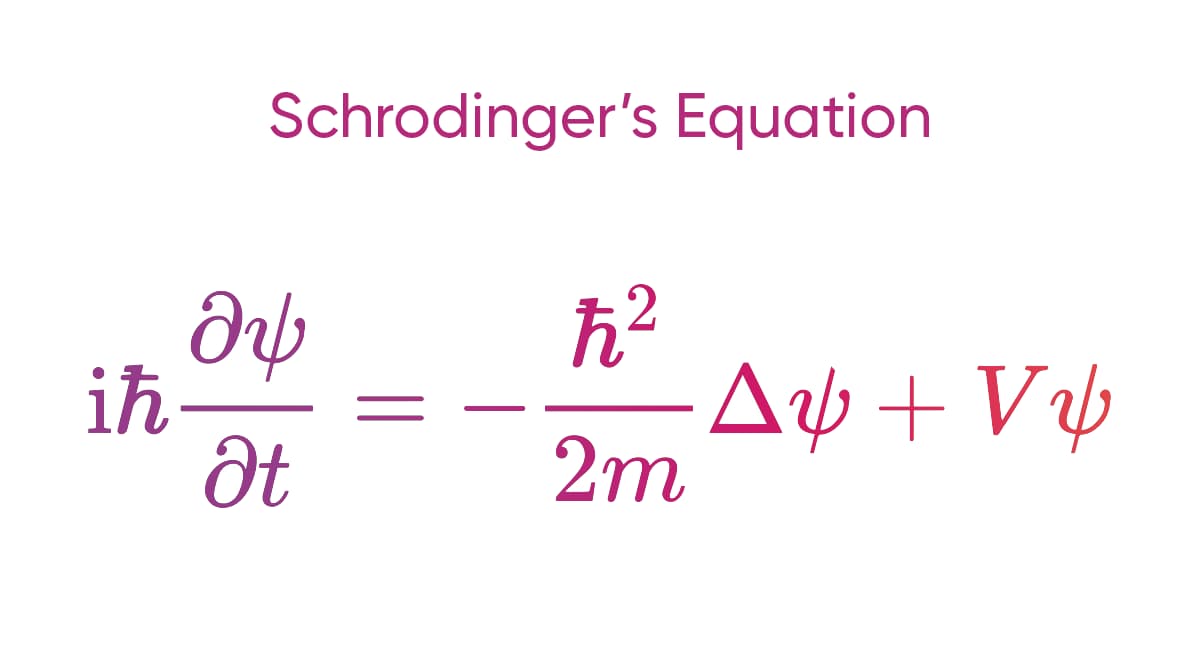
Quantum Mechanics in Everyday Life
While quantum mechanics may initially appear to be an abstract and purely theoretical field, it actually has tangible applications that influence various aspects of our everyday lives. Some examples of how quantum mechanics plays out in daily life are the following:
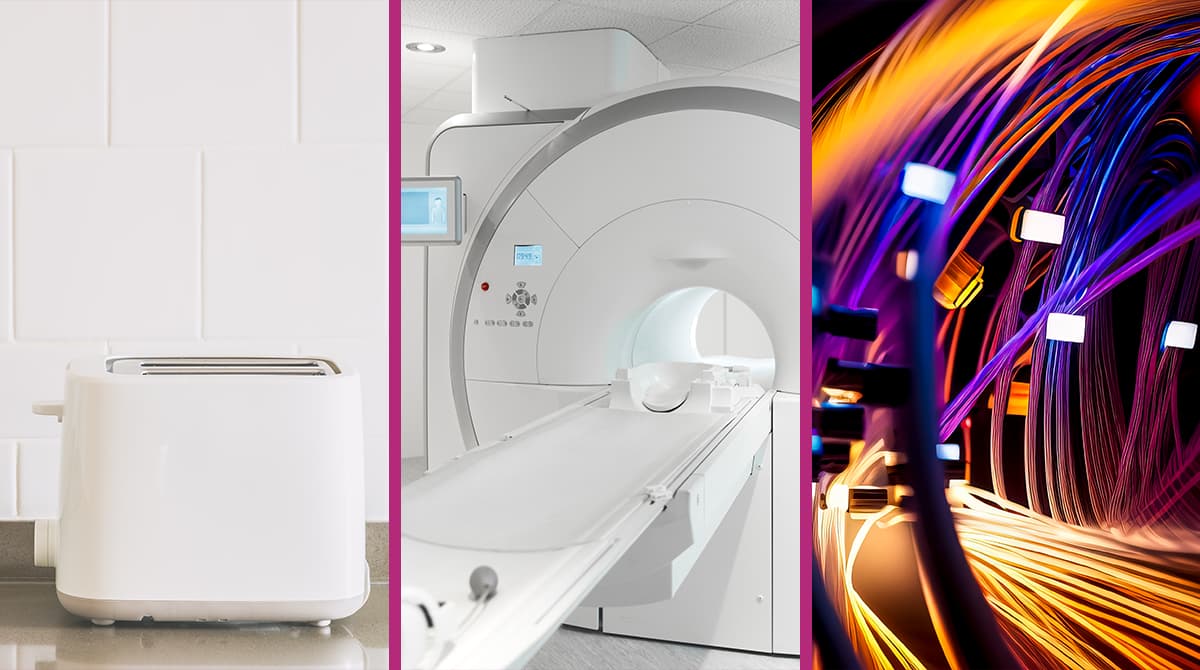
- The design of heating elements in toasters is actually informed by principles of quantum mechanics. Thanks to the behavior of electrons at specific energy levels, both sides of the bread in your toaster get evenly browned.
- Similarly, in fluorescent lamps, the emission of light at particular wavelengths from mercury vapor is also a consequence of quantum mechanics. This allows energy-efficient lighting.
- Transistors, one of the cornerstones of modern technology, are used to amplify electrical signals. These devices were developed based on insights from quantum mechanics.
- The wave nature of electrons is the basis for the operation of semiconductors. These waves enable the development of transistors, the basis of processors in modern computers and mobile devices.
- Quantum mechanics also contributed to the development of electron microscopes. This makes it possible to obtain more detailed images when examining biological samples.
- The Global Positioning System (GPS) relies on atomic clocks, which are fundamentally designed using principles of quantum mechanics.
- Magnetic Resonance Imaging (MRI) operates by reversing the spin of electrons.
- Thanks to quantum mechanics, fiber optic technology was also developed, revolutionizing two-way communication by making it both fast and efficient.
Far from being confined to the realm of subatomic particles, quantum mechanics plays a pivotal role in shaping the reality we experience daily, as well as the larger framework of the universe itself. Much like dark energy, which is invisible yet inferred from its effects on the motion of galaxies, quantum mechanics is interwoven with large-scale cosmological phenomena. This elevates quantum mechanics beyond a mere scientific curiosity; it becomes a key player in our understanding of both everyday life and the broader universe.
What are your thoughts on this matter?
If you had the power over quantum mechanics, the energy of invisible particles, what would you do, what would you invent? We look forward to hearing your ideas.

 Online Services
Online Services Application Inquiry
Application Inquiry Pay Assurance Fee
Pay Assurance Fee Query Installation Number
Query Installation Number Compensation Fee Inquiry
Compensation Fee Inquiry Automatic Payment Order Inquiry
Automatic Payment Order Inquiry Partnership
Partnership






Leave a Comment