What is Ionization? What Does Ionization Energy Mean?
What is in this article?
Ionization energy is a central concept in physics, vital for understanding matter and its behavior. Ionization entails the necessity for energy, crucial for the stabilization of an atom through the process of gaining or losing electrons.
Deeply intertwined with both chemistry and physics, this idea is fundamental to defining the nature of matter. In the scientific realm, the emphasis on ionization energy underscores its importance in ensuring atomic stability.
Continue with the article to explore the nuances of ionization energy, its pivotal role in chemical reactions, and to gain an in-depth perspective on this topic.
What Is Ionization Energy?
Ionization energy is crucial in comprehending atomic structure, shifts in the periodic table, and nuances of chemical reactions. For many, however, it stands as a challenging concept.
Ionization energy is the energy an atom requires to remove its electron from the atom in its ground state. Ionization is a term for atoms attaining stability. This process is fundamental in the realm of chemistry.
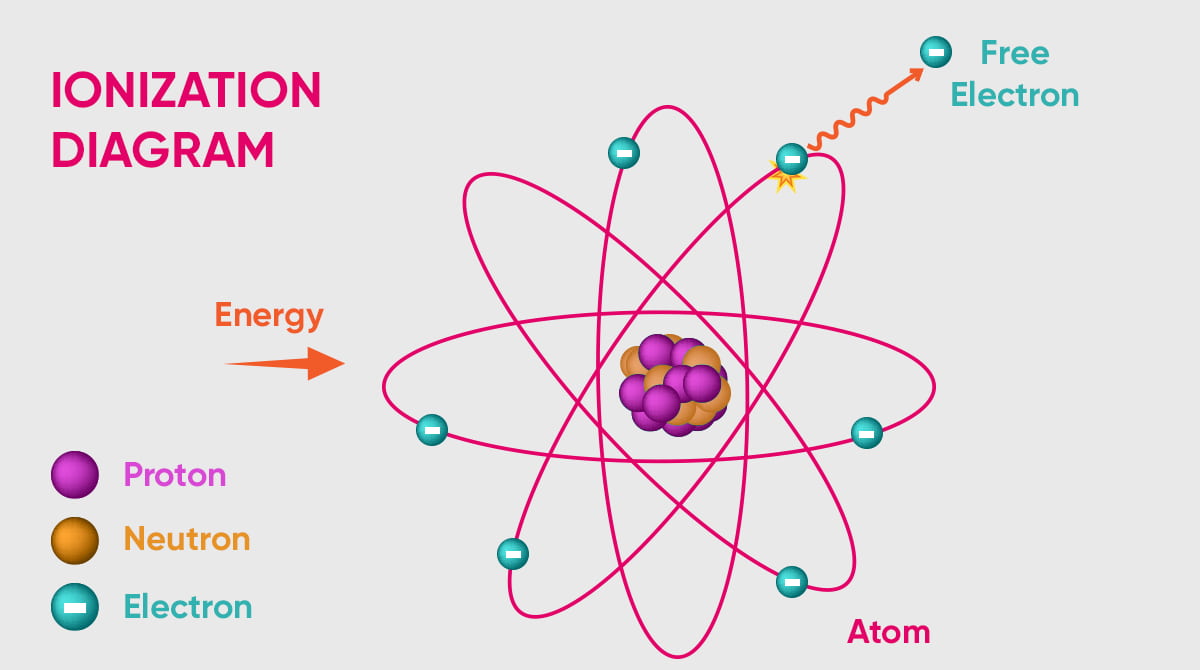
To grasp ionization energy, one must first understand ionization itself. In essence, ionization represents an atom's path to stability. This stabilization is achieved when an atom either donates or accepts electrons. Upon stabilization, the resulting entities are termed ions.
Due to their intrinsic instability, ions often seek interactions with other atoms. This helps them alleviate excess energy. Such interactions primarily involve electron exchanges. The term for this atomic interaction is 'ionization'. The energy threshold necessary for this to occur is what we know as ionization energy.
Delving further, atoms that shed electrons to acquire a positive charge are designated as cations. Conversely, atoms that gain electrons and assume a negative charge are termed anions. When such charged atoms bind, the resulting compounds are identified as ionic bonded compounds.
Understanding why atoms undergo ionization provides insight into ionization energy. Atoms inherently aim to lower their energy states. Their favored method for achieving this is by filling their orbitals (space around the atomic nucleus for electron positioning) to their maximum allowable capacity.
Rare gases are the quintessential examples in this context. These gases, due to their inherent stability, bypass the need for ionization. This intrinsic stability makes them remarkably resistant to forming compounds. Since only 7 of the 188 elements in the periodic table are rare gases, the other 11 elements ionize to become stable.
Ionization energy, denoted by the letter I and quantified in moles, represents the energy alteration when a gaseous neutral atom accrues electrons. In the realm of chemistry, ionization energy stands as a crucial concept and is uniformly endothermic for all atoms. The term 'endothermic' delineates a process where a system siphons energy from its surroundings, typically as heat. Upon imbibing this energy, an atom parts with its electrons, evolving into a positively charged ion.
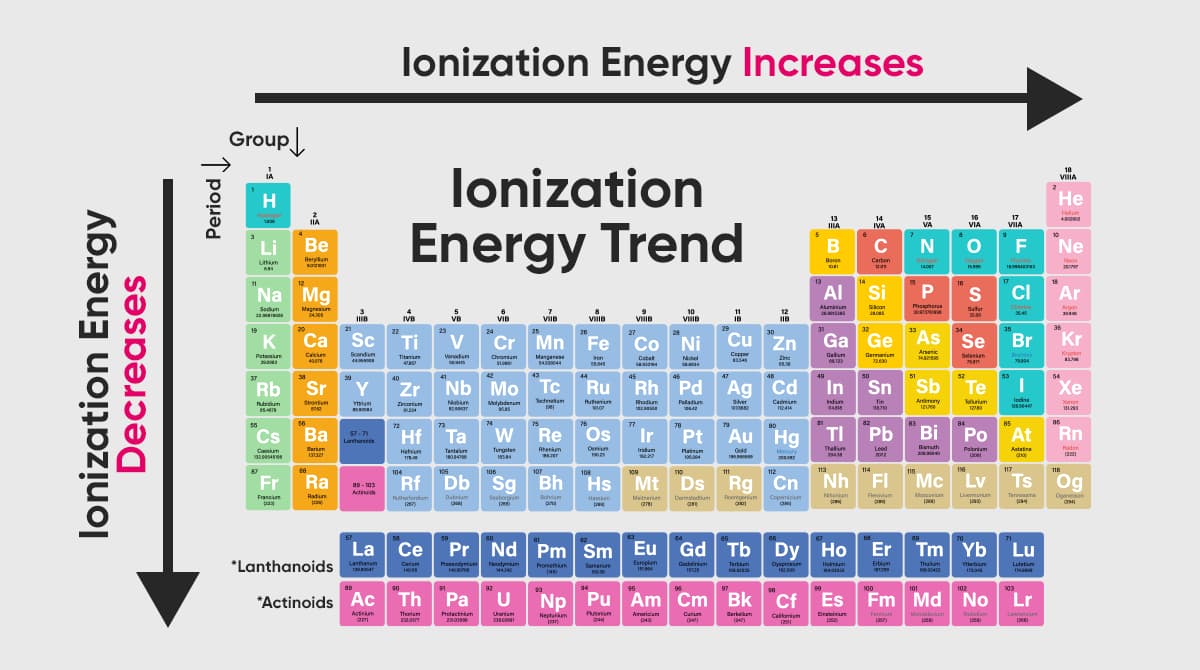
Another definition of ionization energy is a measure of how strongly the electrons in an atom are attracted to the nucleus. Additionally, ionization energy quantifies the energy essential to snap the bond connecting the electrons to the nucleus. One observes a trend in the periodic table: as one traverses from left to right, the ionization energy escalates. This ascent, however, isn't linear.
What Are the First and Second Ionization Energies?
It can be compartmentalized into the first and second ionization energies. The minimum energy an atom demands to spawn an isolated ion in a gaseous state is labeled as the first ionization energy.
Post the extraction of the primary electron, the subsequent energy to liberate the next electron is termed the second ionization energy. After this transformation, what was once a +1 ion evolves into a +2 ion. The second ionization predominantly targets an electron residing in the outermost shell of the element, a connection that's typically weaker than with other elements.
As you traverse from left to right on the periodic table, the first ionization energy typically sees an upward trend. This ascending trend primarily stems from the increasing nuclear charge. Such a charge intensifies the bond of the outermost electron to the nucleus.
Interestingly, there's an inverse correlation between the second ionization energy and the period number. Every atom's ionization energy directly corresponds to its total number of electrons. To illustrate, consider the hydrogen atom. Given its singular elementary structure, its structure is primed solely for the first ionization energy. In contrast, a helium atom, with its pair of electrons, is capable of both the first and second ionization processes.
To distill the concept, the ionization energy of an atom escalates in proportion to its electron count, and this can be summarized thusly:
IE1 < IE2 < IE3 < IE4 < ……
The primary rationale behind this sequencing is that when an electron is removed, the gravitational pull within the nucleus remains unchanged, but there's an amplification in the electromagnetic force. As the electron count dwindles, the electromagnetic force correspondingly intensifies.
The energy requisition for ionization consistently surpasses the preceding one. Consequently, after one electron is extricated, an augmented energy quantum is mandated to liberate subsequent electrons. This succinctly encapsulates the essence of the first and second ionization energies, which facilitate the reaction enabling a neutrally charged atom to acquire either a positive or negative charge.
What Are the Properties of Ionization Energy?
Energy, a cornerstone in both physics and chemistry, denotes the vigor required to undertake a range of actions.
Rooted in the capacity to execute work, the notion of energy is pivotal for the ionization phenomenon. Ionization energy, fostering enhanced efficiency with diminished exertion, facilitates the removal of energy equivalent to 1 mole from an atom in its gaseous state. For acids and bases, ionization translates to their division into positive (+) and negative (-) ions.
The salient characteristics of ionization energy include:
- As one progresses downward on the periodic table, there's an uptick in the energy magnitude.
- The extraction of every mole of electrons corresponds to a minimum energy ionization.
- In the periodic table, the progression of ionization energy across groups is as follows: 1a < 3a < 2a < 4a < 6a < 5a < 7a < 8a.
- Every ionization process for an atom is endothermic in nature.
- Each subsequent ionization event demands a higher energy input than its predecessor. This escalation in ionization energy is denoted as IE1 < IE2.
- For a singular atom, the energies expended to extract the first, second, third, and fourth electrons are respectively termed the first, second, third, and fourth ionization energies.
- The 1st ionization energy is always the smallest.
- The ionization energy of an atom is represented by the reaction: Y(gas) E+1 ---> Y+ e+.
- An inverse relationship exists between ionization energy and atomic size.
- During the ionization process, the count of protons within an atom remains consistent and unaltered.
- Diving into the concept of ionization energy unveils a broader understanding of the periodic table's intricacies.
- Generally, as one navigates from left to right in a given period on the periodic table, ionization energy witnesses an increase.
- Simultaneously, progressing from left to right in the periodic table results in a shrinking atomic radius. A reduced atomic size implies electrons are more tightly bound to the nucleus, necessitating a higher quantum of energy to free them.
- When gauging ionization energies of diverse atoms, the initial criterion is their respective group numbers.
- In instances where atoms have congruent group numbers, their period numbers serve as the distinguishing factor.
The ionization energy serves as a reliable metric to gauge an element's propensity for chemical interactions. These reactions must involve ion formation or electron donation.
It is also important to recognize the ionization energy in order to understand the nature of chemical compounds formed by the combination of elements.
How Does Ionization Energy Increase?
Ionization energy, which is one of the frequently encountered terms in both chemistry and physics, is expressed in different units in the two sciences.
In physics, ionization energy is expressed as EV (electron volts), while in chemistry it is expressed in KJ/mole (molar units).
In the periodic table, as we move from left to right, the ionization energy typically increases. Conversely, within a group, as the size of the atom grows larger, the ionization energy decreases. The reason for the increase in ionization energy from left to right is electrical attraction. The diameters of the atoms become smaller and are therefore bound to the nucleus with a higher attractive force. To break this bond, a higher energy is required.
The number of valence electrons, the base energy level, the electron exchange and the electron affinity are among the factors that increase the ionization energy.
There are some exceptions in the periodic table. For example, the first ionization energy of boron is lower than that of beryllium. Similarly, the first ionization energy of oxygen is lower than the ionization energy of nitrogen. While these instances are outliers, they do not undermine the overall trend.
The way the ionization energy increases is of great interest to researchers in the field of chemical reactions. Ionization energy also increases from bottom to top along the group. Changes in ionization energy provide information about the group number of an element.
Why Do Atoms Ionize?
Atoms ionize to become stable by donating or accepting electrons.
Ionization can occur through collisions with subatomic particles, atoms, molecules, and ions, as well as through magnetic radiation. Other factors that influence the formation of ion pairs include heterolytic bond cleavage and heterolytic substitution reactions. Successful completion of these reactions results in ionization.
Atoms tend to minimize their energy. This is best accomplished by filling their orbitals with a maximum number of electrons. Rare gases do not need to ionize because they are already stable. Other atoms in the periodic table ionize to become stable. For atoms to successfully initiate ionization processes, they must follow the octet rule.
The octet rule is the phenomenon of an atom filling up its last layer with the valence electron. For example, if a layer can carry a maximum of 8 electrons and there are 8 electrons in that layer, the atom is following the octet rule. Atoms prefer shortcuts in this process. If there are 6 electrons in a layer with a capacity of 8 electrons, the atom prefers to take 2 electrons rather than give up 6 electrons.
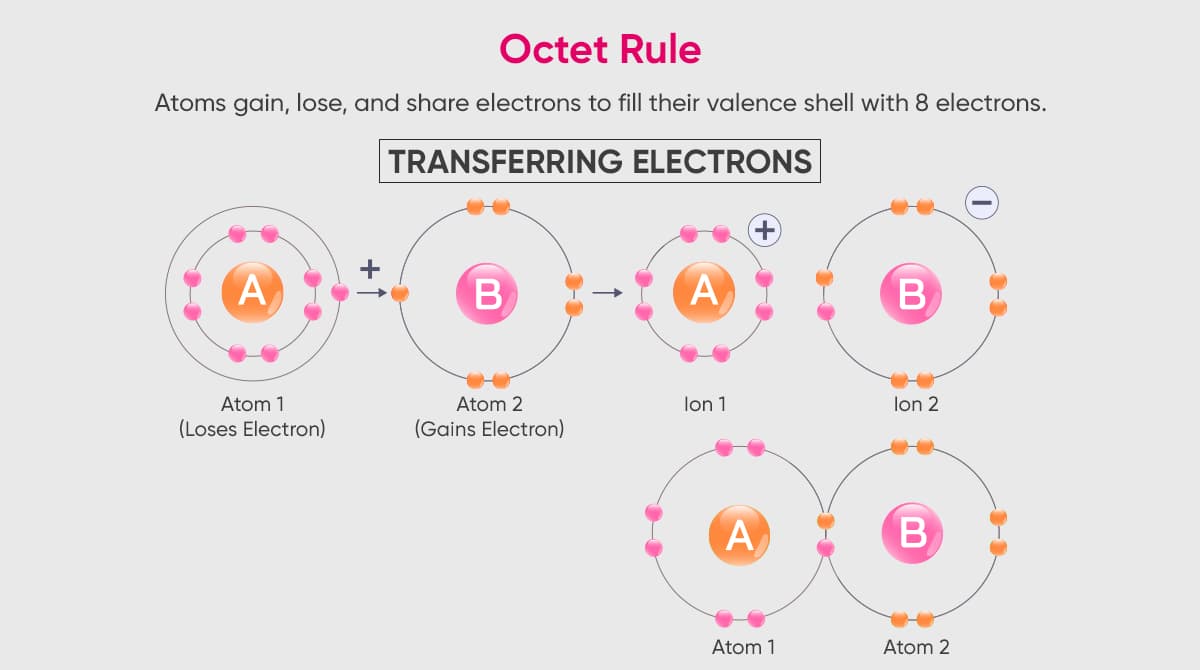
Similarly, an atom with 2 electrons in the last layer chooses to give up 2 electrons rather than take 6 electrons. By giving up 2 electrons, the atom getting rid of its excess charge becomes charged with +2 and becomes stable and this phenomenon is called ionization.
The least amount of ionization energy that the atoms need to carry out all these processes is present in the alkali metals. As the metallicity increases, it becomes equally easier to remove the electrons. For this reason, the ionization energy is low in alkali metals with high metallicity.
The variations in ionization energy, influenced by an atom's position and chemical structure within the periodic table, represent a fundamental area of study in chemistry. Gaining a deeper understanding of chemical reactions and their systematic behavior becomes achievable through familiarity with various chemical formulas, particularly those pertaining to ionization energy.
Feel free to comment on and share this article about ionization energy, a pivotal concept in chemistry, to broaden its reach and inform more individuals.

 Online Services
Online Services Application Inquiry
Application Inquiry Pay Assurance Fee
Pay Assurance Fee Query Installation Number
Query Installation Number Compensation Fee Inquiry
Compensation Fee Inquiry Automatic Payment Order Inquiry
Automatic Payment Order Inquiry Partnership
Partnership




Leave a Comment