
What is in this article?
An inventor in the fields of physics, electricity and chemistry, Alessandro Volta unearthed the aspect of science that arouses curiosity in people. Awarded a special honor by Napoleon, he carved his golden name in the world of science with his many electrical device inventions.
The discovery of electricity has always been fascinating. The contributions of inventors interested in electricity, such as Nikola Tesla, to science paved the way for many future inventions. Listening to his inner inventor, Alessandro Volta pursued his goal of becoming a scientist.
Welcome to a pleasant journey to the world of science by reading our content about the life and inventions of Alessandro Volta, who left his very special mark on human history.
The Life of Alessandro Volta
Born in Como, Italy in 1745, Volta was an important scientist who helped shed light on history. Although Volta came from a noble family, he had financial difficulties due to his father's debts. Despite their difficult times, his parents wanted their son to study and become a distinguished person. His family had to borrow money from their social circles to be able to keep Volta at his education.
As stubborn and determined he was, little Alessandro was also keenly interested in electricity. From an early age, he had a strong desire to advance to new discoveries by observing the results of Alessandro Volta's work.
A hardworking and smart child, Volta had the misfortune of losing his father at seven years old. Unable to get out of the debt from the old era, Volta was forced to move to the Jesuit Boarding School, which trained priests and was free of charge. Finding the education here meaningless, little Alessandro wanted to go to another school.
Where did the Successful Inventor Alessandro Volta Graduate From?
Volta, who loved to deal with science despite his young age, enrolled in Benzi Seminary, the school he always wanted. He continued his education in this school until the age of 18. The Italian physicist, who had ideals, and did not give up his love for science and his ambition to become a scientist, left the Benzi Seminary at the age of 18 and abandoned his education.
Choosing to follow his dreams, Volta started his own scientific studies at the home of his friend Givlio Cesare Gattoni after leaving school. A physicist, Gattoni had set up a physics laboratory in his home.
Volta was able to start experimenting thanks to the physics laboratory in the house of his friend, who was also a physics researcher. While conducting research in the fields he was curious about, he also maintained communication with other scientists through letters. The exchange of information he gained in this way began to broaden his perspective and knowledge of physics.
What was Alexandra Volta's First Invention?
1774 was the year in which Alessandro Volta's determined work bore its first fruits. In 1774, Volta was appointed to the Royal School of Como, where he taught physics. Over the next year, the scientist self-developed rapidly and continued his studies while teaching. The professor relentlessly continued to experiment in the laboratory in the fields of electrochemistry and electromagnetism.
Alessandro Volta's first invention was the electrophorus (generator). However, a similar invention had already been published by Swedish scientist Johan Wilcke. Nevertheless, Volta's invention of static electricity (generator) aroused widespread interest. After his first discovery, the professor grew in confidence and continued to carry out new studies.
Alessandro Volta's Family Life and Invention of the Battery
In 1794 Volta married Teresa Peregrini from Como and started his own family. The scientist, who had three children with Peregrini, a lady of aristocratic roots, wanted to instill his knowledge of physics in his children. For this purpose, he took his children to the laboratory whenever he had time, and told them about the work he had done for the sake of science at a young age.
Alessandro Volta lived a happy family life while continuing his scientific observations. During his studies, Volta reduced the rate of making errors in his new studies by referring to his notebook in which he noted the research results of previous years.
Continuing to improve himself, the physicist had another breakthrough thanks to these notes. He invented the first battery in 1801 and announced this to the world through the press. He paid Abraham Bennet, Tiberius Cavallo and William Nicholsan for helping publicize his discovery.
Having succeeded in making his name widely known, presented his invention to Napoleon at the Institut de France in Paris in 1801. He also gained Napoleon's favor due to his invention of the battery. The famous French ruler recognized Alessandro Volta as a count and presented him with a prize.

What is a Volta Battery?
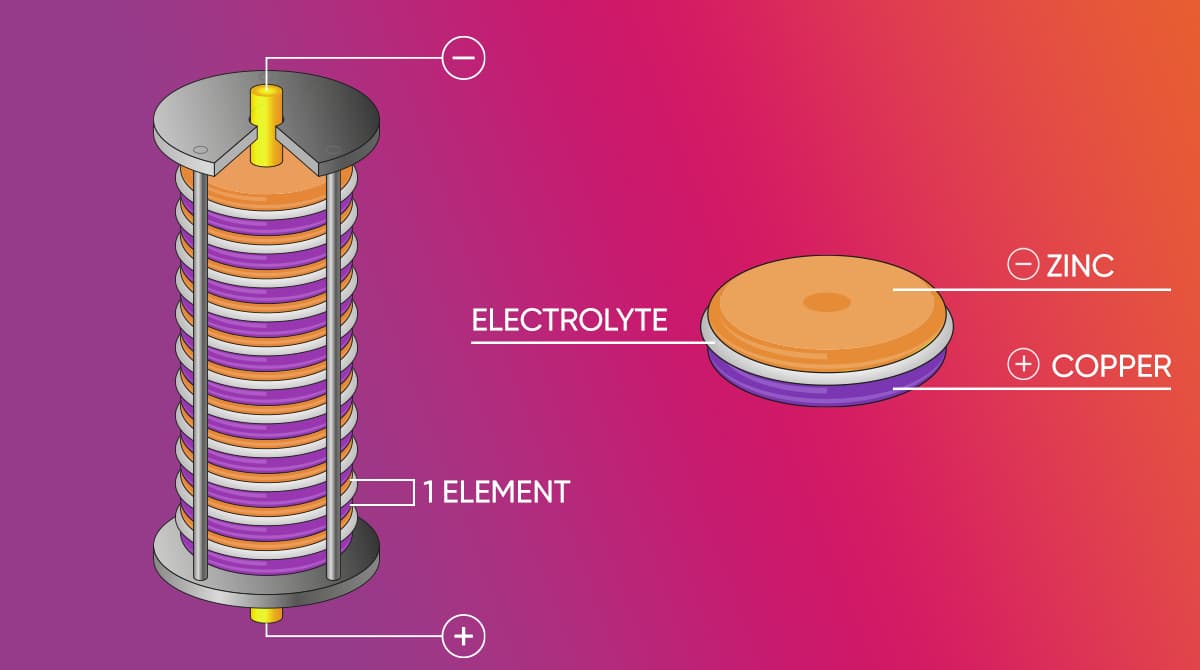
The Volta battery is different from the batteries used today, consisting of pairs of zinc and copper disks stacked on top of each other. Stacked in pairs, the disks were separated by cardboard or cloths soaked in salt water. Invented by Alessandro Volta, the Volta battery was used as one since it could provide continuous electric current to a circuit. One of the important advantages of the battery discovered by the scientist Volta was that it discharged very little when not in use.
One of traits of Alessandro Volta was that he worked tirelessly until he reached the best result. The famous physicist realized that he achieved the most optimal result by using zinc and silver in the structure of the battery he invented. The invention of the Volta battery made way for other discoveries in the scientific world. The most important of these discoveries are:
- The British chemist Humphry Davy discovered how to obtain pure forms of elements.
- Another British chemist William Nicholson used the Volta battery to electrolyze water, which was a chemical decomposition made possible by electric currents.
- Improving on Volta's invention, Andrew Crosse and Humphry Davy were able to increase the level of high voltages.
- Scientific luminaries such as John Frederick Daniell carried Alessandro Volta's battery invention forward, towards its modern structure.
The Relationship Between Luigi Galvani and Volta
Throughout the journey of electricity in human history, many scientists achieved exciting milestones. The Luigi Galvani and Alessandro Volta duo also had a spiraling interest in electricity. Their efforts in the 18th century to understand the nature of electricity gave way to important inventions. The long endeavors by Italian scientists Luigi Galvani and Alessandro Volta finally paid off when they discovered the working principle of electricity. In this way the two scientists contributed to the invention of the battery.

After studying medicine at the University of Bologna, Luigi Galvani became a professor of anatomy. Realizing that electricity could also be generated chemically, Galvani stepped into the world of physics where he made new discoveries.
What pushed Galvani to the field of electricity was that electricity applied to the muscles of a paralyzed person had a stimulating effect that resulted in physical movement. Luigi Galvani thought that this reaction in the human body was related to animal electricity. His idea of experimenting on a dead frog, that is, touching two different types of metal to the legs of a dead frog yielded physical reflexes, which prompted him to coin the Animal Electricity theory. Based on his observations, Galvani concluded that there is internal electricity in living things.
On the contrary, Volta believed that the reflex produced in the frog's leg was a result of artificially generated, external electricity. Working to come up with his own evidence, like Galvani himself, Volta thought of touching his tongue with different types of metals.
This resulted in a little electric shock when he touched his tongue with mercury and copper at the same time. Thanks to this famous experiment, Volta discovered that the source of electricity is external. Hence Alessandro Volta was able to invent the battery with contributions from Luigi Galvani's animal electricity thesis.
Inventor of the Volta battery, Alessandro Volta proved that electricity could be chemically generated, thereby debunking the knowledge that electricity was generated via living beings. The Volta battery left its mark on the world of science, stimulating an increase in innovations in the field of electrochemistry.
How Does a Battery Work?
A battery is a device which stores chemical energy that is converted into electrical energy. Non-rechargeable batteries have a primary structure, while long-lasting rechargeable batteries are built on a secondary structure. Disposable, that is, non-rechargeable, batteries are used in products such as flashlights, toys and radios. However, rechargeable batteries are generally preferred for devices with high energy consumption.
A battery has different parts depending on the type. The way a battery works is better understood with the help of concepts of electron attraction and electrodes. The power unit in a battery is named a cell. Consisting of three main elements, a cell envelops electrolyte and two electrodes. It is also a matter of safety precaution to enclose these three main elements of the cell in plastic or metal sheaths.
The two electric terminals are marked with a plus and a minus. These marks are connected to the electrolytes within the battery. When these two electrolytes are connected to the circuit, the electrolyte starts receiving signals. The chemical reaction between ions and the electrolyte resets the chemical called electrolyte, which depletes the battery.
While the history of the battery spans back to Luigi Galvani, Alessandro Volta laid the foundations for the modern battery. Also, Thomas Edison announced the discovery of the iron-nickel battery as a result of his work. The most widely used battery type today is the lithium-ion battery, which was produced by Gilbert Newton Lewis in 1912.
Other Inventions of Alessandro Volta
The idea of communicating with scientists who were well-known and successful in the world of science led Volta to go to Switzerland in 1777. Having made valuable friends, Volta met Horace Bénédict de Saussure, who, like him, was fond of research. He also had a close friendship with the renowned geologist H.B. and Sausurre.
After his Switzerland trip, Volta returned to his laboratory to resume his studies, all the while maintaining his habit of reading. Benjamin Franklin's work titled Flammable Air in the field of physics had a deep impact on Volta. Adding activities in the field of chemistry to his scientific repertoire in physics, the scientist started studying the chemistry of gases.
The experience he gained in the field took him to the discover of coal gas in 1776, further raising his already renowned name in the world of science. Adding one more to his discoveries, Volta was appointed professor of experimental physics at the University of Pavia in 1779. While here, he continued his studies in physics for 40 years.
After his discovery of methane, the famous physicist noticed in November 1776 that it was readily present in the Maggiore River. Professor Volta also studied capacitors. Pieter Van Musschenbroek had invented the capacitor, which creates storage space for electric charge. However, it was Volta who improved the capacitor and made it widespread in 1775.
How to Calculate Volts
Volt is the unit used to measure voltage. Voltage, represented by the symbol "V", is technically a unit of potential energy difference between two points in an electrical circuit. Volts measure the amount of voltage flowing through electricity or a circuit, which help understood the voltage requirements of electrical power needed in different applications.
Calculating Volts requires command of Ohm's law. Often used in electrical calculations, Ohm's law establishes the relationship between voltage, conductor resistance and current. It is referenced in computer, electronic and electric circuit analyses. The voltmeter, that is, the formula to be used in the calculation of Volts is as follows:
V= I x R
V (Volts: Voltage) = I (Ampere: Current) x R (Ohm: Resistance)
This formula is used to evaluate the desired volt measurement.
Volts can be measured with a multimeter or voltmeter. The parallel connection system, which is added to the circuit together with the voltage stage, enables the measurement. Volt meters make it easy to measure the voltage intensity of different types of fields.
Every device that is part of our daily lives or that we use carries the memory of Alessandro Volta. Which piece of information about the inventor of the battery interested you the most? Don't forget to share with us in the comments. If you want others to know how the battery was invented, share our blog content on social media platforms!

 Online Services
Online Services Application Inquiry
Application Inquiry Pay Assurance Fee
Pay Assurance Fee Query Installation Number
Query Installation Number Compensation Fee Inquiry
Compensation Fee Inquiry Automatic Payment Order Inquiry
Automatic Payment Order Inquiry Partnership
Partnership






Leave a Comment